Airtightness Testing for Medical Devices
WAFU Brothers plays a critical role in the field of airtightness testing within the medical industry by providing high-precision testing equipment. Their technology is applied in **medical device seal testing**, ensuring the safe operation of ventilators, anesthesia machines, and more. It is also used in **pharmaceutical packaging seal testing** to prevent drug contamination. Additionally, the equipment supports airtightness testing for **implantable medical devices**, **respiratory masks**, and **dialysis equipment**, ensuring patient safety. With its advanced technology, WAFU Brothers offers reliable support to the medical sector and has become a key partner in the industry.
Trocar for Laparoscopy
Critical Channel for Minimally Invasive Surgery
A key channel for delivering endoscopes or surgical instruments into the body cavity during minimally invasive procedures.
Seal Failure Risk
CO₂ gas leakage can reduce pneumoperitoneum pressure, limiting the surgical field of view.
Regulatory Compliance
Poor sealing may fail to meet medical device standards (e.g., EN ISO 80369, YY/T 0466).
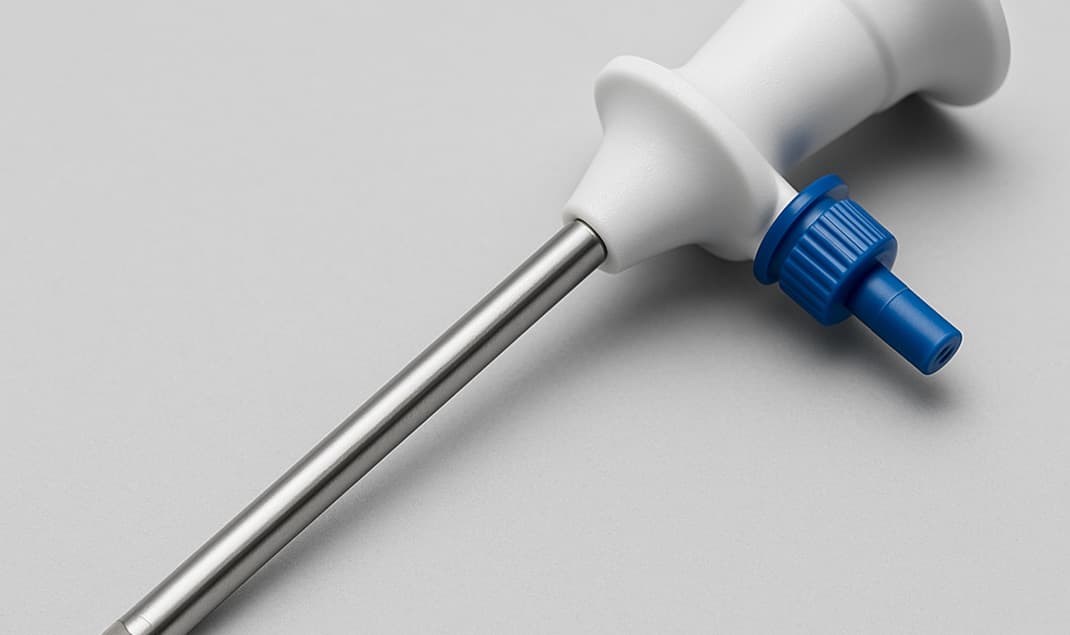
Medical-grade Sealing Assurance
Ensure trocar sealing, enhancing surgical safety and success rate
Digital Blood Pressure Monitor
Measurement Accuracy
Leakage reduces pressure and results in inaccurate blood pressure readings.
Extended Device Lifespan
Continuous leakage forces the pump to overwork, shortening the device lifespan.
Medical Certification
Non-compliance with YY 0670, ISO 81060 and other regulations may hinder market access.

High-precision Leak Detection
Ensure airtightness of the monitor for accurate results
Vial Bottles
Drug Stability Assurance
As sterile packaging for injectables, sealing quality directly affects drug stability, safety, and shelf life.
Contamination Risk Prevention
Microbial or moisture intrusion may degrade or contaminate the drug solution.
Global Regulatory Compliance
Failure to meet sealing requirements under USP <1207>, YY0033 etc. may block export or registration.
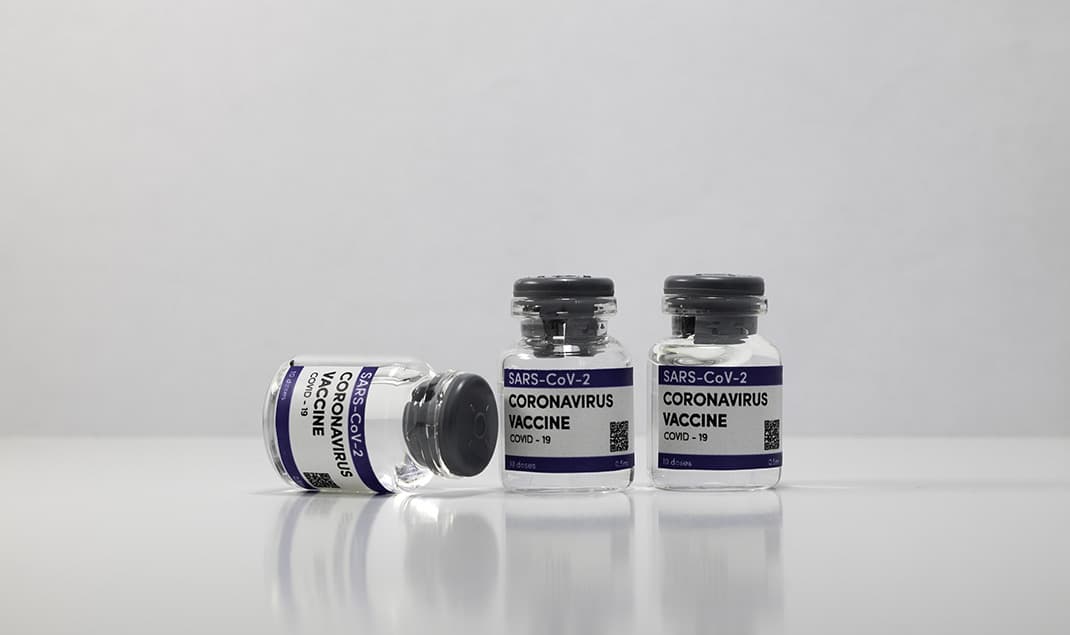
Sealing Inspection for Drug Packaging
Ensure vial sealing integrity to protect drug quality and safety
 WAFU
WAFU